Flowering plants, or angiosperms, represent more than 95% of all vascular plant life on Earth, exhibiting unmatched ecological, functional and morphological diversity. While they presently dominate nearly all terrestrial environments, the early origins of flowering plants remain an enigma. Unequivocal angiosperm fossils are not known until the Cretaceous period, where an abrupt but enormous diversity of evidence appears. In fact, the apparently rapid and tremendous evolutionary diversification of angiosperms in the Cretaceous was the great “abominable mystery” of Darwin and many of his contemporaries1. Molecular clock dating has frequently suggested an earlier origin for angiosperms in the Jurassic (or even Triassic) period, such as that performed by Zeng et al.2. However, many studies have lacked extensive sampling, especially at the ordinal and familial levels. Now, in a new paper published in Nature Plants, Li et al.3 describe a comprehensive angiosperm phylogeny sampling nearly 3,000 chloroplast (plastid) genomes from species representing all 64 orders and 85% of the 416 flowering plant families. Molecular clock dating using 62 fossils as calibration points supports the earlier origin of flowering plants dating the crown group to the Late Triassic period (~209 million years ago (Ma)). This earlier age leaves a gap between the earliest fossils and the origin for flowering plants of almost 70 million years, roughly the entirety of the Jurassic period, and is coined by the authors as the ‘Jurassic angiosperm gap’.
In their study, Li et al.3 compiled all angiosperm chloroplast genome data from the National Center for Biotechnology Information database, added data for 677 species from the OneKP Project4 and generated 1,659 new chloroplast genomes, totalling 2,881 genomes. From these genomes they selected 80 genes, including the exons of nearly all chloroplast protein-coding and ribosomal RNA genes. Using a maximum likelihood framework, the authors generated a chloroplast angiosperm tree of life with high statistical support for most relationships. Nearly all of the evolutionary relationships described by their phylogenetic assessment are in agreement with the angiosperm phylogeny group (APG) version IV summary tree, with some differences in a few key relationships. For example, the mainly leafless and achlorophyllous Petrosaviaceae family (with a complicated taxonomic history) emerged as sister to orchids with high statistical support — a position entirely different from its placement in the APG IV tree. Further, many relationships that were ambiguous in previous analyses are described in the phylogeny of Li et al.3 with strong statistical support.
The earliest lineages of flowering plants are shown by Li et al.3 to originate in the Late Triassic and Early Jurassic. These early lineages are represented by comparatively few numbers of species totalling a mere couple hundred extant taxa, including the New Caledonian Amborella trichopoda, water lilies (Nymphaeales) and the woody plant order Austrobaileyales, which contains star anise. Li et al.3 demonstrate that major diversifications subsequently occurred in the Late Jurassic and Early Cretaceous resulting 99.95% of the extant diversity of flowering plants. The relationships of the five major clades of this large diversification (core angiosperms) have long been difficult to determine2, and their resolution remains a challenge here, despite the use of 80 genes.
A Triassic beginning for angiosperms corresponds with the timing of the origin of some insect groups, including crickets and katydids, alderflies and the common ancestor of caddisflies — moths and butterflies5. However, the spectacular diversification of core angiosperms in the Jurassic and Early Cretaceous notably also coincides with the origin and evolution of Phytophaga, arguably the most diverse radiation of plant-feeding beetles6. Their association with angiosperms has long been proposed to account for the apparent evolutionary success of Phytophaga7. Moreover, modern beetle diversity in general as well as other pollinators — including moths and butterflies — had their origins in the Cretaceous, which coincides with the rise of flowering plants to ecological dominance and the major diversifications of extant angiosperm diversity in the phylogeny of Li et al.3.
This new study is not without limitations since molecular dating analyses tend to report older ages for groups than the earliest accepted fossils for them, leading some to suggest, and occasionally demonstrate that, molecular dating is biased toward older age estimates8. Still, the dates from this study fall within the range of most of the recent estimates for angiosperms despite different methods and sampling, which perhaps suggests a robustness to them. Another point is that the phylogeny of Li et al.3 is solely based on chloroplast data and would be strengthened by an analysis with nuclear genes. To say this is difficult in plants is an understatement given the rampant history of polyploidy and genome duplications. These processes, which are of great interest to evolutionary biologists, make the identification of genes of common ancestry for phylogenetics challenging. Still, new experimental methodologies9 and analytical advances10 are improving our ability to carry out robust phylogenetic analyses in the face of polyploidy and genome duplications.
So, why the Jurassic gap? One explanation is that in the early days, flowering plants were sparse and thus fossils are rare and presumably difficult to locate and identify. Another explanation is perhaps they occurred in environments that were unsuitable for preservation. Darwin himself suggested, though calling it “wretchedly poor conjecture”, that angiosperms could have had a pre-Cretaceous history on a remote, but lost, island, meaning that all evidence of early flowering plants was essentially wiped out1. A somewhat less exotic explanation is that the structures, flowers or fruits of early flowering plants were too small to now be confidently assigned to angiosperm lineages. Whatever the story, the Jurassic angiosperm gap remains a mystery. The new study by Li et al.3, however, presents a comprehensive framework to further investigate its causes and to investigate the evolution and remarkable rise to dominance of flowering plants (Fig. 1). (Nature Plants)
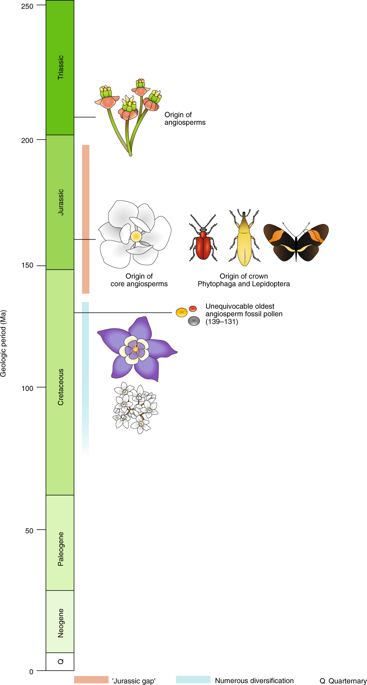
The findings of Li et al.3 support an older age for the timing of the origin of flowering plants in the very late Triassic (~209 Ma). This date is substantially earlier than unequivocally assigned angiosperm fossils, which appear beginning in the Cretaceous (~139–132 Ma). According to the time-calibrated phylogeny of Li et al.3, core angiosperms originated in the Jurassic and their date coincides with the radiation of the plant-feeding beetle group Phytophaga6 as well as Lepidoptera (moths and butterflies)5. Subsequent to this, numerous diversifications of flowering plants occurred in the Cretaceous (~140–90 Ma).




