The genus Salvia, one of the biggest genera in the medicinally important family Labiatae, is a rich source of diversity diterpenoids with attractive bioactivities such as tanshinone IIA, salvinorin A, and salvicine, et al. Many species of this genus such as S. miltiorrhiza, S. trijuga, S. yunnanensis, S. przewalskii, and S. prionitisare used as folk medicines to treat a wide variety of ailments.
Previous systematic studies about the chemical constituents of Salvia plants by XU Gang et al. have resulted in the isolation of manyterpenoids with diverse structures derived from normal abietane diterpenoid skeletone. (J. Agric., Food Chem., 2010, 58, 12157-12161; J. Chromatogr. A, 2009, 1216, 4847-4858; Tetrahedron, 2008, 64, 9490-9494; Org. Lett., 2007, 9, 291-293; Org. Lett., 2006, 8, 4453-4456. et al.). Recent investigation about the terpenoid constituents of S. przewalskii have resulted in the isolation of przewalskone (1), an unprecedented adduct of a danshenol type terpenoid and an icetexane diterpenoid via a hetero-Diels-Alder cycloaddition. Its structure and absolute configurations were determined on the basis of extensive analysis of NMR spectra and crystal X-ray diffractions. Compound 1 exhibited significant cytotoxicities against five human cancer lines in vitro (IC50 0.69 ~ 2.35 μM).
A plausible biogenetic pathway for 1 was also proposed. It evident that hetero-Diels-Alder cycloaddition could account for the formation of compound 1 from two different moieties. The first moiety was a derivative of danshenol type terpenoids which can be seen as one of the three major types of C23 terpenoids. It’s noteworthy that C23 terpenoids, consisted of not more-than thirty members, are really a small group of metabolites with diverse structures derived from normal diterpenoids. To the best of our knowledge, only four compounds have been reported to possess a danshenol type C23 terpenoid skeleton so far. For the second moiety, less than ten icetexanes featured with a 6/7/6 carbon rings system derived from normal abietane diterpenoids have been reported since 2000 from the literature research.
The study has been published online of Chemical Communications. (http://pubs.rsc.org/en/content/articlelanding/2012/cc/c2cc30405h).
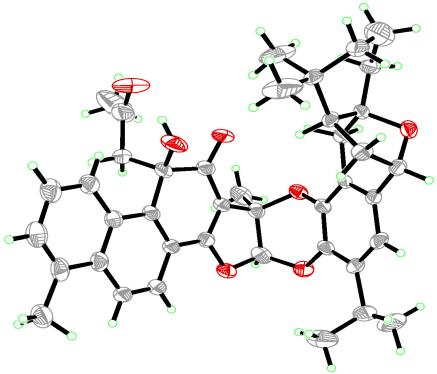
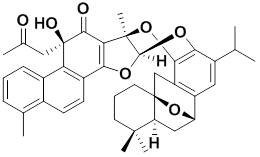
The structure of przewalskone (1) (image by KIB)






