Carotenoids are important pigments that are involved in photosynthesis as well as the sole source of pre-vitamin A. Dietary carotenoids have been shown to be beneficial to health by decreasing the risk of many diseases. Astaxanthin (3,3'-dihydroxy-β-carotene-4,4'-dione) is a unique red carotenoid responsible for the pigmentation of many marine animals which acquire the red pigment from their diets not only for coloring but also for improving survival and growth of juveniles. Growing evidence also shows that astaxanthin provides beneficial effects on human health, including the enhancement of general well-being and the immune system, protection against lipid-membrane peroxidation and DNA damage. While chemically synthetic astaxanthin is restricted in use as a feed additive for salmon and trout farming, the demand for natural astaxanthin is expected to grow rapidly in the nutraceutical, cosmeceutical and pharmaceutical markets. However, the bioresources of astaxanthin are limited and thus expensive, which has attracted scientists to develop heterologous biosynthesis hosts for the sustainable production of natural astaxanthin. However none of these attempts has led to satisfactory yields of astaxanthin.
Recently a research group led by HUANG Junchao’s group , Kunming Institute of Botany, has achieved significant progress on astaxanthin biosynthesis in higher plants. They isolated and characterized several algal β-carotene ketolases (BKT) and found that the BKT from Chlamydomonas reinhardtii could confer plant cells to efficiently synthesize astaxanthin (Zhong et al., 2011; Huang et al., 2012). Furthermore, they have generated a more nutritious tomato by modifying the intrinsic carotenes to astaxanthin by introducing the algal BKT gene into the genome of a B-type tomato (Huang et al., 2013). Massive accumulations of mostly free astaxanthin in leaves (3.12 mg/g) but esterified astaxanthin in fruits (16.1 mg/g) were found in the transgenic tomato, leading to a 16-fold increase of total carotenoid capacity in the fruits with above 80% of the carotenoids being astaxanthin. This achievement opened up the possibility of employing crop plants as green factories for economical production of astaxanthin.
Huang J.C, Zhong Y.J, Liu J,Sandmann G, Liu J and Chen F (2013) Metabolic engineering of tomato for high-yield production of astaxanthin. Metabolic Engineering. http://dx.doi.org/10.1016/j.ymben.2013.02.005.
Huang J.C, Zhong Y.J, Sandmann G, Liu J and Chen F (2012) Cloning and selection of carotenoid ketolase genes for the engineering of high-yield astaxanthin in plants. Planta 236:691-699.
Zhong Y-J, Huang J.C *, Liu J, Li Y, Jiang Y, Xu Z-F, Sandmann G, and Chen F (2011) Functional characterization of various algal carotenoid ketolases reveals that ketolating zeaxanthin efficiently is essential for high production of astaxanthin in transgenic Arabidopsis. Journal of Experimental Botany 62:3659-3669.
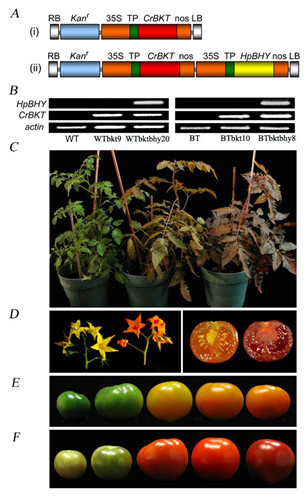
Phenotypes of T1 generation tomatoes over-expressing the CrBKT or HpBHY-CrBKT genes.
(A) Schematic diagram of the T-DNAs in the two plasmids used to transform tomato. (i) The T-DNA consisted of a selectable marker (npt׀׀) cassette (Kanr), the 35S promoter (35S) from cauliflower mosaic virus, C. reinhardtii bkt coding sequence (CrBKT) functionally fused to the tomato RUBISCO chloroplast transit peptide (TP), with a nos terminator. (ii) This T-DNA resembles construct (i) with an extension of an additional cassette comprising the H. pluvialis BHY coding sequence fused to the tomato TP.
(B) RT-PCR detection of CrBKT and HpBHY expression in transgenic plants.
(C) B-type tomato plant (left) and its CrBKT transformant (TB-b10, center) and its CrBKT-HpBHY transformant (TB-bb8, right) showing different color in leaves;
(D) Phenotypes of B-type flowers (left), TB-b10 (right) and cross-section of ripe B-type (left) and TB-bb8 (right) fruit;
(E) and (F) fruits at different ripening stages of B-type (E) and transgenic plant TB-bb8 (F).




