Alternaria alternata (tobacco pathotype) is a necrotrophic fungus that infects mature tobacco leaves and causes the so called Brown-spot disease which results in severe losses in Nicotiana species.
Until now most studies about plant-pathogen interaction have either focused on the regulating roles of several phytohormone pathways such as JA, SA, ET which are commonly strongly activated after fungal attack, or investigated secondary metabolites like scopoletin and capsidiol and their antimicrobial functions after infections. However, connections between plant’ signaling and the induction of secondary metabolites were greatly lacking.
Prof. WU Jinsong and his master student SUN Huanhuan from Kunming Institute of Botany found that young leaves of wild tobacco Nicotiana attenuata were more resistant to A. alternata than mature ones, and this was correlated with stronger blue fluorescence (indicating the accumulation of certain defense metabolites) induced after infection. However, the nature of the fluorescence-emitting compound, its role in defense and regulation were not clear. Silencing feruloyl-CoA 6’-hydroxylase 1 (F6’H1) the key enzyme gene for scopoletin biosynthesis by virus-induced gene silencing (VIGS) revealed that the blue fluorescence was largely emitted by scopoletin and its ß-glycoside form, scopolin. Further analysis showed that scopoletin exhibited strong antifungal activity against A. alternata in vitro and in vivo. Importantly, JA levels were highly elicited in young leaves but much less in mature ones after infection and fungus-elicited scopoletin was abolished in JA deficient plants, but largely restored with methyl jasmonate treatments. Consistently, plants strongly impaired for JA biosynthesis and perception were highly susceptible to A. alternata. Furthermore, silencing MYC2, a master regulator of most JA responses, reduced A. alternata-induced NaF6’H1 transcripts and scopoletin. Thus, Prof. Wu concluded that JA signaling is activated in N. attenuata leaves after infection, which subsequently regulates scopoletin biosynthesis for the defense against A. alternata partly through MYC2, and higher levels of scopoletin accumulated in young leaves account for their strong resistance.
The story was published under the title: “Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on JA signaling” in the Journal of Experimental Botany. All details can be found at http://jxb.oxfordjournals.org/content/early/2014/05/08/jxb.eru203.abstract?keytype=ref&%2520ijkey=C6RHVyIz3RwQLW7. The project is supported by the Chinese Academy of Sciences, and the Scientific Research Foundation of the Ministry of Education of China for Returned Scholars to WU Jinsong .
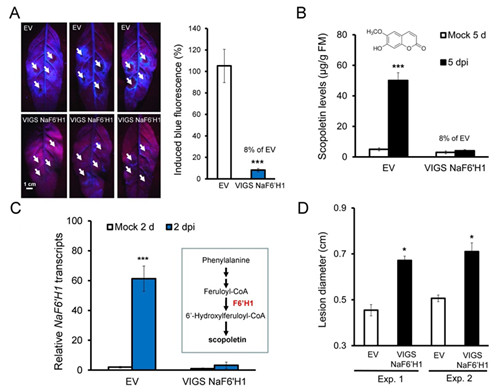
Fig.1 Silencing NaF6’H1 dramatically reduced A. alternata-elicited blue fluorescence and scopoletin, and plant resistance
(A): Left: three independent young leaves from EV and VIGS NaF6’H1 plants were infected with A. alternata. Photographs were taken from their backside under UV light at 5 dpi, strong blue fluorescence was emitted around the four inoculation sites (pointed by white arrows) of each leaves in EV but not in VIGS NaF6’H1 plants. Right: quantification of blue fluorescence intensity induced by A. alternata at 5 dpi in 4 replicated leaves of EV and VIGS NaF6’H1 plants. The level of the intensity in EV plants was arbitrary set as 100%. Asterisks indicate the level of significant differences between EV and VIGS NaF6’H1 plants (Student’s t-test: *** p<0.005).
(B): Mean (± SE) scopoletin levels were determined by LC-MS/MS in 5 replicated young leaves of EV and VIGS NaF6’H1 infected with A. alternata for 5 d. Asterisks indicate the level of significant differences between mock and infected samples in EV plants (Student’s t-test: *** p<0.005).
(C): Mean (± SE) relative A. alternata-induced NaF6’H1 transcript levels as measured by real-time PCR in 4 replicated leaves of EV and VIGS NaF6’H1 plants at 2 dpi. Inset: schematic description of scopoletin biosynthetic pathway. Asterisks indicate the level of significant differences between mock and infected samples in EV plants (Student’s t-test: *** p<0.0001).
(D): Mean (± SE) diameter of necrotic lesions in 4-replicated young leaves of EV and VIGS NaF6’H1 infected with A. alternata for 7 d. Two independent VIGS experiments were presented showing similar results. The asterisk indicates the level of significant difference between EV and VIGS NaF6’H1 leaves (Student’s t-test: *, P<0.05).
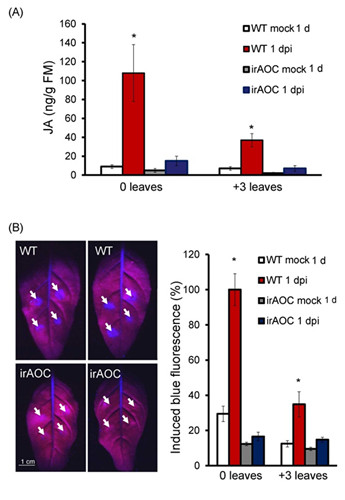
Fig.2 A. alternata-induced JA is required for the fungus-elicited scopoletin production.
(A): Mean (± SE) JA levels elicited by A. alternata at 1 dpi determined by LC-MS/MS in 5 replicated 0 and +3 leaves of WT and irAOC plants.
(B): Left: two 0 leaves from WT and irAOC plants were infected with A. alternata for 1 d. Photographs were taken from their backside under UV light, strong blue fluorescence was emitted around four inoculation sites (pointed by white arrows) of each leaves in WT plants but not in irAOC plants. Right: means (± SE) relative blue fluorescence intensity induced by A. alternata at 1 dpi in 5 replicated 0 leaves of WT or irAOC plants. The level of the intensity in 0 leaves of WT plants was arbitrary set as 100%. Asterisks indicate the level of significant differences between mock and infected samples (Student’s t-test: * p<0.05).
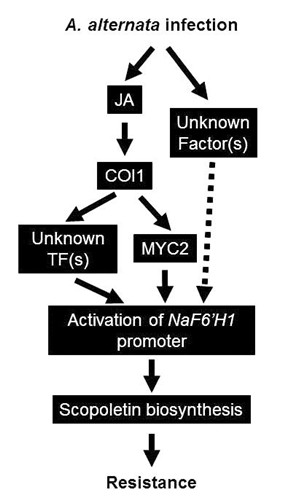
Fig. 3 Working model of the regulation of A. alternata-induced scopoletin production by JA signaling.
After A. alternata infection the JA signaling pathway is activated and NaF6’H1, coding for the key enzyme in scopoletin biosynthesis, is trans-activated by the binding of MYC2 and some other unknown proteins to the T/G-box of the NaF6’H1 promoter; the biosynthesis of scopoletin around the infection sites finally affects the resistance of N. attenuata to A. alternata. However, without A. alternata elicitation MeJA treatment itself could not induce scopoletin production, indicating that another JA-independent signal is also needed for NaF6’H1 activation.




