Polycyclic polyprenylated acylphloroglucinols (PPAPs) are a special class of complex natural products that have only been isolated from plants of the family Guttiferae so far. Biogenetically, the acylphloroglucinol cores are presumably derived from a characteristic polyketide-type biosynthesis and their prenylation is realized through an enzyme-catalyzed addition to afford monocyclic polyprenylated acylphloroglucinols (MPAPs), which may be further cyclized to PPAP type metabolites with diverse carbon skeletons. These kinds of metabolites showed a wide variety of biological activities such as antitumor, antimicrobial, anti-HIV, antioxidant, and antidepressant activities. In recent years, many novel PPAPs with unique skeletons have been reported, and their fascinating chemical structures and intriguing biological activities have attracted increasing attention from phytochemical, organic synthetic, and pharmacological fields.
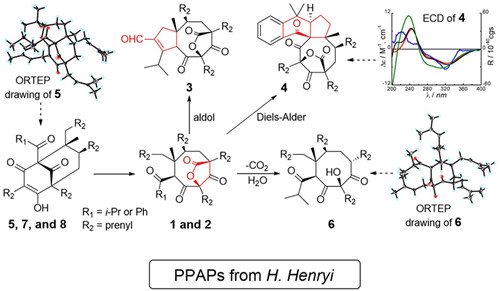
Hypericum henryi is a traditional Chinese medicinal plant used for the treatment of hepatitis in Yunnan. In this study, hyphenrones A–F, six PPAP type derivatives with four architectures including three unprecedented cores were isolated from this plant by Dr. XU Gang’s group at Kunming Institute of Botany. Their absolute configurations were defined by experimental and calculated ECD spectra and the X-ray diffractions coupled with the presumed close relationship in their biosynthetic pathway. hyphenrones A and B possess an unprecedented seco-PPAP skeleton formed by cleavage of the C-1/C-9 bond of the normal endo-BPAPs. hyphenrones C and D, featuring fascinating 5/8/5 and 6/6/5/8/5 fused ring systems, were presumably synthetically derived from hyphenrones A and B via key aldol condensation and Diels-Alder cycloaddition reactions, respectively. This work was published on Organic Letters. (http://pubs.acs.org/doi/abs/10.1021/ol500808p) and also been selected as “Hot off the Press” by “Nat. Prod. Rep.” (DOI: 10.1039/c4np90023e).
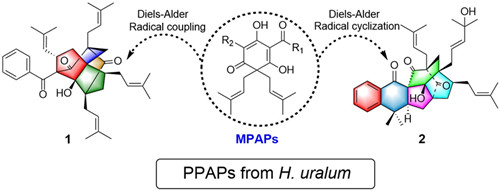
Hypericum uralum, a perennial shrub mainly distributed in Tibet and northwest of Yunnan, P. R. China, was not phytochemically studied previously. In the systematic study of the PPAPs of H. uralum by Dr. XU Gang’s group, a polycyclic polyprenylated acylphloroglucinol possessing an unprecedented tetracyclo-[5.3.1.14,9.04,11]-dodecane core (Hyperuralone A), was characterized from H. uralum together with hyperuralone B, a congener with another complex caged skeleton. Their structures were determined by extensive spectroscopic analysis and ECD calculations. A plausible biosynthetic pathway of their intriguing architectures via intramolecular Diels–Alder reactions was also proposed. Presently, this study has been published on Organic Letters. (http://pubs.acs.org/doi/abs/10.1021/ol502425f), and selected as “highlight” by the journal (http://pubs.acs.org/journal/orlef7).