Thrombotic disease is one of the major causes of human death. Unfortunately, almost all anticoagulants currently used could cause severe
The intrinsic tenase, theoretically, is the final and rate-limiting enzyme complex in the intrinsic pathway of the blood coagulation cascade. It is an attractive but less explored target for developing new anticoagulants due to the lack of pure selective inhibitors. Interestingly, a series of oligosaccharides with potent and selective inhibitory activities for intrinsic tenase are firstly purified by Prof. ZHAO Jinhua’s group from Kunming Institute of Botany (Proc. Natl. Acad. Sci. USA, www.pnas.org/cgi/doi/10.1073/pnas.1504229112). The purified oligosaccharides particularly inhibit intrinsic pathway, while have little effects on extrinsic and common pathways of the blood coagulation cascade, highlighting a great interest for the development of the compound(s) in treatment of the thrombotic disease.
In their report, compound 5 is a smallest compound among these oligosaccharides that have strong anti-tenase activity. The compound demonstrates significant antithrombotic activity as strong as clinically most commonly used drug, Enoxaparin, in rats’ venous thrombosis model. Importantly, the compound has no significant effect on hemostatic function even at high dose, whereas Enoxaparin causes significant hemorrhagic tendency, demonstrating a potential better usage in development of the drug in near future. Besides, the finding is highly valuable for clarifying the difference between physiological hemostasis and pathological thrombosis.
The oligosaccharides are obtained from the fucosylated glycosaminoglycan (FG) which is a natural product extracted from sea cucumber but the precise structure of native FG has not been elucidated for years due to the difficulty in chemical researches of complicated polysaccharides. Additionally, FG has non-selective intrinsic tenase inhibitory activity, but its application is limited for its activation of F.XII and platelet. Prof. ZHAO’s group has devoted on investigating the chemical structure of FG and their structure-function relationship of the inhibition for tenase, and has progress in depolymerization of FG by selective glucosidic bond cleavage (Gao N, Lu F et al. Carbohydr Polym, 2015, 127, 427–437; Zhao LY, Lai SS et al. Carbohydr Polym, 2013, 98, 1514–1523), chemical modification (Gao N, Wu MY et al. Mar Drugs, 2012, 10, 1647–1661; Lian W, Wu MY et al. Biochim Biophys Acta, 2013, 1830, 4681–4691) and structure-function relationships of FG (Wu MY, Wen DD et al. Eur J Med Chem, 2015, 92, 257–269) since 2010. Now, ZHAO Longyan and WU Mingyi et al. firstly report a series of oligosaccharides compounds prepared from FG by selective glucosidic bond clevage and purification technique, and elucidate the precise chemical structure of complicated FG by structure analysis of purified oligosaccharide compounds.
This article contains supporting information online at http://www.pnas.org/content/early/2015/06/17/1504229112.full.pdf
This work was funded in part by the Yunnan Provincial Science and Technology Department in China (2010CI116, 2013FA046 and 2012FB177), National Natural Science Foundation of China (81373292), Outstanding Technical Talent Foundation and West Light Foundation of the Chinese Academy of Sciences.
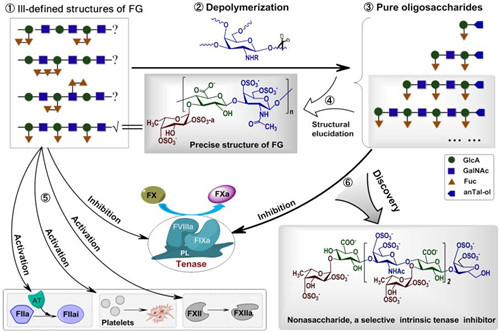
Figure 1. The doubts on the detailed structures of native FG 1 exist for over 30 years. By depolymerization of FG with selective glucosidic bond (GalNAc β1→) cleavage 2 and structure analysis of purified oligosaccharides 3, the precise chemical structure of FG is firstly elucidated 4. Native FG has various pharmacological activities on thombosis 5 (e.g. AT-dependent antithrombin activity, platelet and F.XII activation and Tenase inhibition), while the pure oligosaccharides (nona-, dodeca-, pentadeca- and octadecasaccharides) can selectively inhibit intrinsic tenase 6.
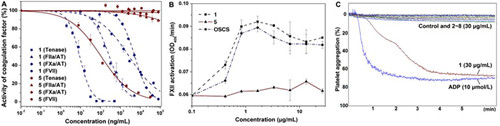
Figure 2. Effects of native FG (1) and pure nonasaccharide (5) on intrinsic tenase activity and FVIIa, FXa, and FIIa activities in the presence of AT (A), FXII activation (B), and platelet aggregation (C).
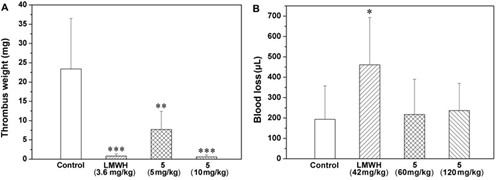
Figure 3. Effects of the pure nonaccharide (5) on inferior vena cava thrombosis of rats (A) and bleeding of mice (B).
Contact:
ZHAO Jinhua
State Key Laboratory of Phytochemistry,Kunming Institute of Botany
E-mail:zhaojinhua@mail.kib.ac.cn




