Natural products with architecturally distinct scaffolds remain attractive for the discovery of potential drugs and biological probes. To collect new compounds with privileged skeletons, however, is becoming more challenging. Through various combinations of chemical and biosynthetic approaches, large numbers of structurally interesting and biologically active natural product analogues have been generated to focus on a common scaffold in each case. On the other side, biosynthetic machineries usually accommodate divergent pathways to produce a dazzling array of architecturally distinct skeletons from only a handful of basic building blocks.
Prof. ZENG Ying and Prof. LIU Jikai’ teams at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences (CAS) investigated how structural diversity is expanded from relatively few fundamental blocks is therefore necessary to promote the development of creative approaches for the skeletal diversification of compounds. With this aim in mind, research attention was captured by the basidiomycete fungus Boreostereum vibrans (syn. Stereum vibrans). ( http://onlinelibrary.wiley.com/doi/10.1002/anie.201208182/abstract).
Based on recent elucidation of the biosynthetic pathway for vibralactone, research demonstrated a divergent pathway to skeletally distinct meroterpenoids in B. vibrans from a common intermediate that has a shikimate-terpenoid origin. Moreover, a FAD-binding monooxygenase VibMO1 was identified that converts prenyl 4-hydroxybenzoate into prenylhydroquinone and is likely involved in the biosynthesis of vibralactone G and other meroterpenoids in B. vibrans. The pathway to 3-substituted γ-butyrolactone such as vibralactone G is distinct in starting from an aryl ring of shikimate origin, which is unusual and the first time such a pathway has been described in natural product chemistry.
This study affords in vivo and in vitro verification of oxidative decarboxylation of prenyl 4-hydroxybenzoate and, subsequent to this, the important discovery of the first genetic and biochemical characterization of a FAD-binding monooxygenase VibMO1 that converts prenyl 4-hydroxybenzoate to prenylhydroquinone and is likely involved in the biosynthesis of meroterpenoids in B. vibrans fungus.
This identification of VibMO1 will also be informative for the determination of enzymes essential for similar conversion steps in the biosynthesis of yeast coenzyme Q and plant meroterpenoids, where the oxidative decarboxylation of (poly)prenyl hydroxybenzoates to their hydroquinones is understood to occur yet no corresponding genes or enzymes have so far been described.
This study also demonstrates divergent biosynthetic pathways toward distinct scaffolds from a single precursor. To create skeletal diversification of compounds, further insight into nature’s strategy for expanding structural diversity is needed.
The study has recently been published in Angewandte Chemie International Edition 2016, 55: 5463–5466 (http://onlinelibrary.wiley.com/doi/10.1002/anie.201510928/full).
This work was supported by the grants from the National Natural Science Foundation of China (21572237, 81561148013).
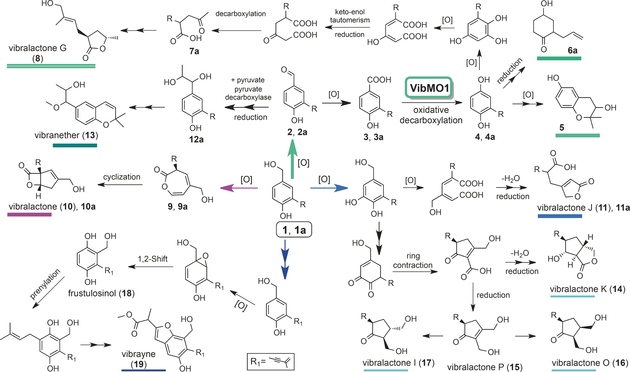
Figure 1: One to all and all from one -- a divergent pathway for vibralactones and other meroterpenoids in normal and 1a feeding broths of B. vibrans. R=allyl for analogues with compound designations containing a; R=prenyl for natural products (Image by KIB)
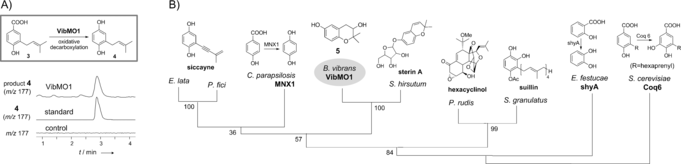
Figure 2: A) LC/MS analysis of the VibMO1 activity by single-ion monitoring of [M−H]− at m/z=177 for the enzymatic product 4. B) Phylogenetic connection of VibMO1 (GenBank: KU668560) and its fungal homologues. The tree scale was modified to accommodate the chemical structures (see Section S2). Shown at the top of the tree are the corresponding prenyl(hydro)quinone derivates or the verified enzymatic reactions of each fungus (Image by KIB)
Contact:
State Key Laboratory of Phytochemistry and Plant Resources in West China
Kunming Institute of Botany, Chinese Academy of Sciences
Prof. ZENG Ying
E-mail: biochem@mail.kib.ac.cn




