In 2016, Prof. YANG Yurong’s group at Kunming Institute of Botany, Chinese Academy of Sciences (KIB/CAS) reported the first asymmetric total synthesis of two monoterpenoid indole alkaloids Alstoscholarisine A and Aspidophylline A in the flagship journals of chemistry- Journal of the American Chemical Society (JACS) and Angewandte Chemie International Edition(ACIE ) simultaneously, which attracted the attention of the synthetic community. At the beginning of 2017, the same team just published the first catalytic asymmetric synthesis of Communesin F, a star molecule with complex indole structure, in the JACS.
The communesins share an identical synthetically challenging heptacyclic core arrayed with two aminal groups and at least five stereogenic centers, including two vicinal quaternary carbons. This combination of intriguing structural complexity and interesting biological activities renders these alkaloids popular synthetic targets. It is noteworthy that two Chinese famous chemists have made significant contributions in the field. In 2007, QIN Yong reported the inaugural synthesis of (±)-communesin F. In 2010, MA Dawei completed the first asymmetric synthesis as well as the assignment of the absolute configuration of (−)-communesin F. Other three chemists Weinreb, Funk and Movassaghi have also published the wonderful syntheses.
Completing such a difficult molecule as Communesin requires an ingenious design of the novel synthetic strategies. Based on the analysis of the existing five synthetic routes, YANG Yurong et al. designed an efficient synthetic route to solve the key issues. An unprecedented iridium-catalyzed asymmetric intermolecular cascade cyclization, constructing the lower N,N-aminal-containing CDEF tetracyclic core in one step, was developed. Another notable element is the closure of final ring system (A ring) via a facile reduction of a twisted amide and concomitant cyclization activated by mesylation of N,O-hemiaminal intermediate.
The research paper titled as “Ir-Catalyzed Asymmetric Total Synthesis of (−)-Communesin F” was favorably commented by three reviewers and has been published online in theJournal of the American Chemicial Society ( http://pubs.acs.org/doi/abs/10.1021/jacs.7b00854)
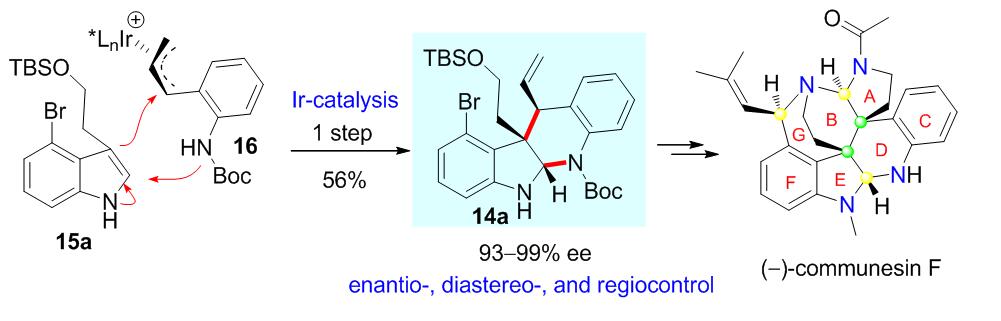
Fig.1 (Image by KIB)
This work was financially supported by the Strategic Priority Research Program of the CAS (Grant No. QYZDB-SSW-SMC026) and the NSFC (21672224, 21472200).
Contact:
State Key Laboratory of Phytochemistry and Plant Resources in West China
Kunming Institute of Botany, Chinese Academy of Sciences
Prof. YANG Yurong
E-mail: yangyurong@mail.kib.ac.cn.




