Maturation drying is a pivotal step during seed development in many plant species. Once dry, and after dispersal from the parent plant, the seeds enter a relatively quiescent state. Favourable environmental conditions thereafter facilitate germination. Prolonging seed lifespan by drying has also enabled humans to conserve genetic resources and provide high-quality planting material for the future.
Seed desiccation tolerance means the survival ability after seed drying, has scientific significance and value. The acquisition of desiccation tolerance and mechanism is the hot and difficult issue in seed biology for a long time.
The percentage of non-tropical plants producing desiccation-sensitive seeds has been estimated at 7% to 25% and this proportion increases to up to 48% in the wet tropics. Given that many species that produce seeds that are not fully desiccation-tolerant are commercially important trees or shrubs [e.g. Theobroma cacao, Quercus sp, some coffee (Coffea) and Citrus], it is important to understand both the mechanistic basis of seed desiccation stress and to develop innovative conservation practices for the seeds of such species.
The physiological aspects of seed recalcitrance have been reported extensively for a wide range of species over the last four decades. Researchers know little about its cellular process and molecular basis. Although clustering of species with seeds that are highly desiccation sensitive is known within certain families, simple taxonomic or significant phylogenetic predictors are not evident, until now the molecular mechanism of seed desiccation tolerance still unclear.
By profiling membrane lipids of seeds in eight species (representing around nearly 20% of orders represented in the Core Eudicots) with varying levels of desiccation tolerance, Dr. CHEN Hongying and Professor LI Weiqi from Kunming Institute of Botany, Chinese Academy of Science (KIB/CAS) together with Professor Hugh Pritchard from Royal Botanic Gardens Kew found a close association between reducing seed viability and increasing phosphatidic acid (PA).
The team applied hydration-dehydration cycles to Arabidopsis seeds, that are normally desiccation tolerant, to mimic the onset of desiccation sensitivity with progression towards germination and examined role of phospholipase D (PLD) in desiccation stress-induced production of PA.
This research found that PLDa1 became more abundant and migrated from the cytosol to the membrane during desiccation, whereas PLDd did not change, and that all desiccation-induced PA was derived from PLDa1 hydrolysis. When PLDa1 was suppressed, the germination level after each hydration-dehydration cycle improved significantly.
Furthermore, this study demonstrated that PLDa1-mediated PA formation modulates desiccation sensitivity as applying its inhibitor improved seed desiccation tolerance and its suppression in protoplasts enhanced survival under dehydration. The insights provided by comparative lipidomics enable researchers to propose a new membrane-based model for seed desiccation stress and survival.
During drying, relatively more extensive changes in lipid composition occur in seeds that are less tolerant of desiccation, a response that is mediated by the migration of PLDa1 from the cytosol to the membrane and the production of PA. PA accumulates both in axis and cotyledon, is extremely damaging when levels in the membrane exceed 6%, possibly through a mechanism of phase separation and destabilisation of the membrane bilayer.
During or after maturation and shedding, the cytoplasm of both desiccation tolerant and sensitive seeds have the potential to enter the glassy state but maturation drying in planta of orthodox seeds enables a regulated entry into the glassy state, and sooner than that of recalcitrant seeds. PLDa1-mediated PA formation is hindered and ion leakage is blocked once the cytoplasm has entered the glassy state. Shorter exposure times and smaller increases in PA mean that orthodox seeds successfully avoid this form of cumulative membrane damage. In contrast, failure of desiccation sensitive seeds to deter a massive PA accumulation during unregulated metabolism during water loss has profound consequences for cell survival.
This study has recently been published in Plant, Cell and Environment (a top 5% journal in plant science) entitled "Phospholipase Dα1-mediated phosphatidic acid change is a key determinant of desiccation-induced viability loss in seeds". http://onlinelibrary.wiley.com/doi/10.1111/pce.12925/full
It was supported by the National Nature Science Foundation of China and the Gempalsm Bank of Wild Species.
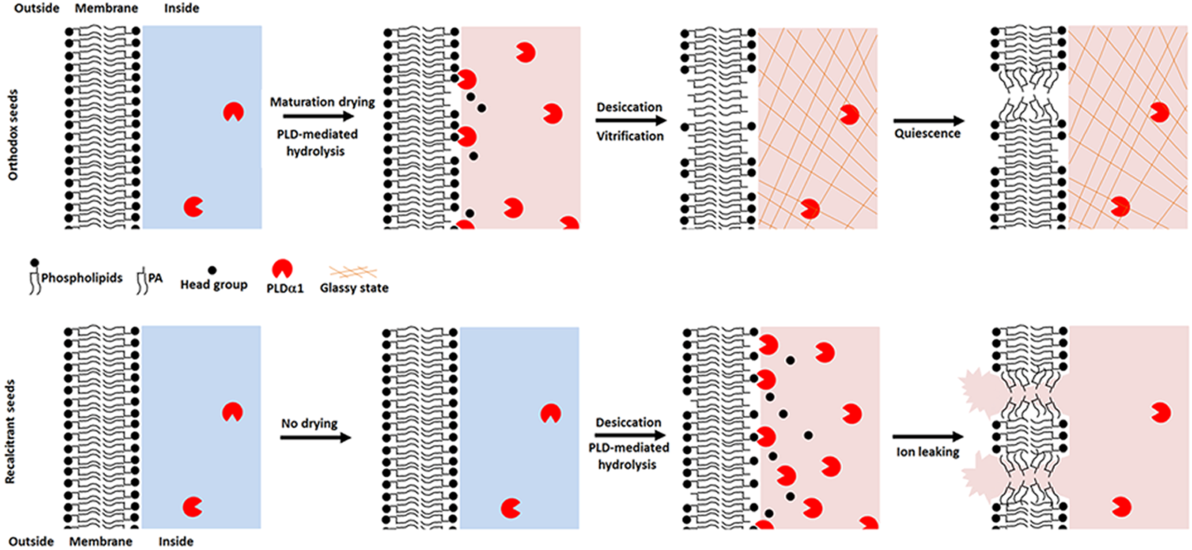
Fig. 1: A working model of cellular processes and molecular mechanism for determining seed storage behaviour.
Contact:
Prof. LI Weiqi
Germplasm Bank of Wild Species
Kunming Institute of Botany, Chinese Academy of Sciences
Email: weiqili@mail.kib.ac.cn




