Carotenoids are yellow to red pigments synthesized by all photosynthetic organisms. Lutein, β-carotene, and zeaxanthin are essential carotenoids for human health, serving as provitamin A or retina pigments. Human and animals have to obtain these carotenoids from their diets.
In contrast to lutein and β-carotene that can derive from marigold flowers and the green alga Dunelialla salina respectively on large scales, no commercial sources of natural zeaxanthin are available up to date.
Recently, the research team led by professor HUANG Junchao from Kunming Institute of Botany, Chinese Academy of Sciences (KIB/CAS) isolated a generally recognized as safe (GRAS) and carotenoid-producing bacterium, Sphingobium sp..
This GRAS bacterium was exploited as a cell factory for high-yield production of zeaxanthin. First, whole-genome sequencing and analysis of pathway genes were applied to define the carotenoid pathway in Sphingobium sp.. Secondly, a Sphingobium transformation system was established to engineer metabolite flux into zeaxanthin.
By a combination of chemical mutagenesis and removal of bottlenecks of carotenoid biosynthesis via overexpression of three rate-limiting enzymes, they developed a genetically modified Sphingobium strain named DIZ, which could produce 21.26 mg/g DCW of zeaxanthin, about 4-fold higher than the WT strain.
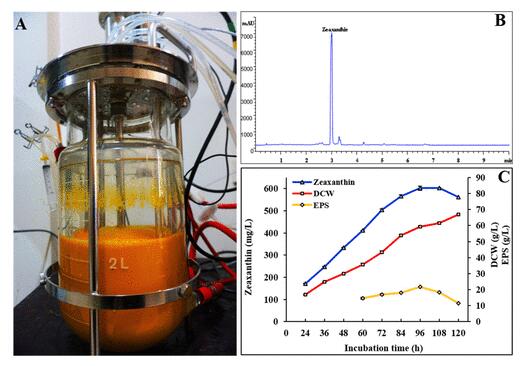
Furthermore, after optimization of culture conditions, the DIZ strain produced 479.5 mg/L of zeaxanthin with the productivity of 4.99 mg/L/h and 21.9 g/L of exopolysaccharides using a fed-batch fermentation strategy.
This study represents the first genetic manipulation of Sphingobium sp., a biotechnologically important bacterium, for high-yield production of value-added metabolites.
The results were published in Journal of Agricultural and Food Chemistry with the title “Enhanced coproduction of cell-bound zeaxanthin and secreted exopolysaccharides by Sphingobium sp. via metabolic engineering and optimized fermentation”.
This study was funded by a research grant from Yunnan Key Laboratory for Wild Plant Resources.

Contact:
YANG Mei
General Office
Kunming Institute of Botany, CAS
email: yangmei@mail.kib.ac.cn
(Editor:Yang Mei)




