Parasitic plants are extraordinary plants with unique physiology, ecology, and evolutionary histories. Little is known about their origin and evolution. 
Initially, certain autotrophs evolved to be facultative hemiparasitic plants which obtained only water and mineral nutrients from their hosts as supplements.
Some of the facultative hemiparasitic plants later became obligate parasitic plants that had to depend on their hosts to complete their life cycles.
Gradually holoparasitic plants evolved from obligate parasites and they completely lost their photosynthesis capacity.
In angiosperms, parasitic plants independently evolved 12 or 13 times. In eight parasitic plant lineages, hemiparasitic species became extinct, leaving only holoparasites remaining. However, the Orobanchaceae (Lamiales), the largest family of parasitic plants, comprises autotrophic and parasitic plants with all degrees of parasitism. This makes it by far the best family for studying the origin and evolution of plant parasitism
Recently, a research team led by Prof. WU Jianqiang from the Kunming Institute of Botany, Chinese Academy of Sciences (KIB/CAS) sequenced and annotated the reference genomes of orobanchaceous plants, the autotroph Lindenbergia luchensis and two economically important holoparasitic pests Orobanche cumana and Phelipanche aegyptiaca. (Figure 1).

Figure 1. Plant species in the family Orobanchaceae spanning from non-parasitic to increasing levels of parasitism. (Image by KIB)
By combining these newly sequenced genomes with existing data, researchers performed the largest comparative genomics analysis of parasitic plants to date, revealing the evolution of the orobanchaceous parasitic genomes.
The researchers found that unlike dodder, orobanchaceous parasitic plants did not undergo a dramatic contraction in genome size and gene number; instead, both hemiparasitic and holoparasitic orobanchaceous plants had larger genomes and more genes than the autotrophic Lindenbergia luchensis.
The haustorium is an unique organ in parasitic plants that enables them to establish a physical connection with their hosts so that they can live parasitically.
Using haustorium transcriptome data, the researchers identified haustorially highly expressed genes in the genomes of various parasitic plants and found that these genes also underwent dramatic expansions.
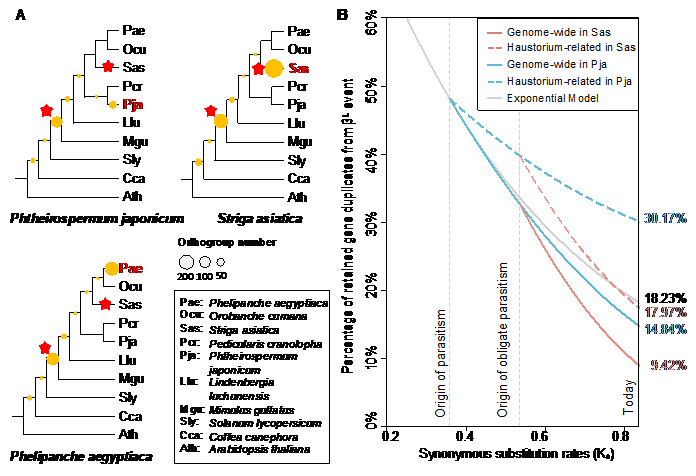
There have two notable periods of gene expansions, one caused by a paleopolyploidy event ( βL event, about 73.48 million years ago, before the emergence of parasitism), and another was caused by an independent duplication after the divergence of each parasitic plant. Comparison with the neutral model revealed that the βL event played an important role in the origin of the haustorium, although it happened about 35 million years before the origin of parasitism (Figure 2).

Figure 2. The role of the ancient WGD event βL in the emergence and evolution of parasitism in the Orobanchaceae.(Image by KIB)
Gene family evolutionary analysis revealed that these parasitic plants underwent simultaneous gene family contraction and expansion in their genomes. Gene loss became more severe as the degree of parasitism increased: from the about 2-3% gene loss in the facultative hemiparasites to about 6-7% gene loss in obligate hemiparasites, and to about 13-15% gene loss in holoparasites.
More than half of the genes lost in the Orobanchaceae were identical to those lost in dodder, a parasitic plant with a completely different origin. Most of these genes were related to photosynthesis and chloroplast function.
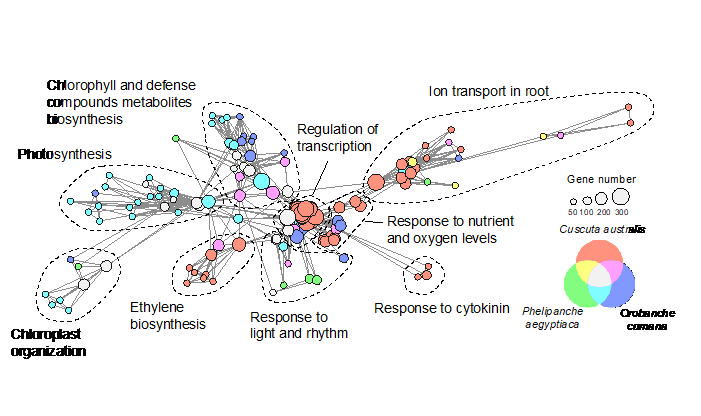
The differential gene loss was mainly related to the two different parasitic forms, stem parasitism and root parasitism, with orobanchaceous plant losing more genes related to photosynthesis as well as light signaling, while dodder losing more genes related to root organ function (Figure 3).

Figure 3. Gene loss in the orobanchaceous parasitic plants and Cuscuta australis. (Image by KIB)
The research supports a three-phase model of parasitic plant genome evolution: first, development of haustorium which enabled a physical connection with host plants; second, after the haustorium had evolved, the parasites started to lose genes that were no longer needed, and third, the parasites evolved to adapt to their specific hosts.
This study has been recently published in Molecular Plant, entitled “Comparative genomics of orobanchaceous species with different parasitic lifestyles reveals the origin and stepwise evolution of plant parasitism
This research was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program of Chinese Academy of Sciences, the CAS "Light of West China" Program, the Special Research Assistant of Chinese Academy of Sciences, and the Postdoctoral Directional Training Foundation of Yunnan Province.
(Editor: YANG Mei)




